Risk considerations
Ethics considerations and risk levelling
Whilst ethical risks can be difficult to identify, knowing how to identify them is important. When designing and planning your research you need to consider who is involved in your study, and how their participation might benefit them and your research. The risk infographic ‘Ethics: Risk Considerations’ highlights potential areas of ethical issues that might be relevant for a given research study.
Each bubble represents a different risk category, each interconnected and some with overlap. One should not be reflected upon in isolation, and each can carry risks that need to be mitigated or managed. This includes risks and consequences for:
- individual researchers
- research participants
- individuals, groups, communities connected either with the research participants or the research topic
- the reputation of Queen Mary and its researchers.
When completing the QMEthics application it is important to identify the risks and explain clearly how to mitigate them.
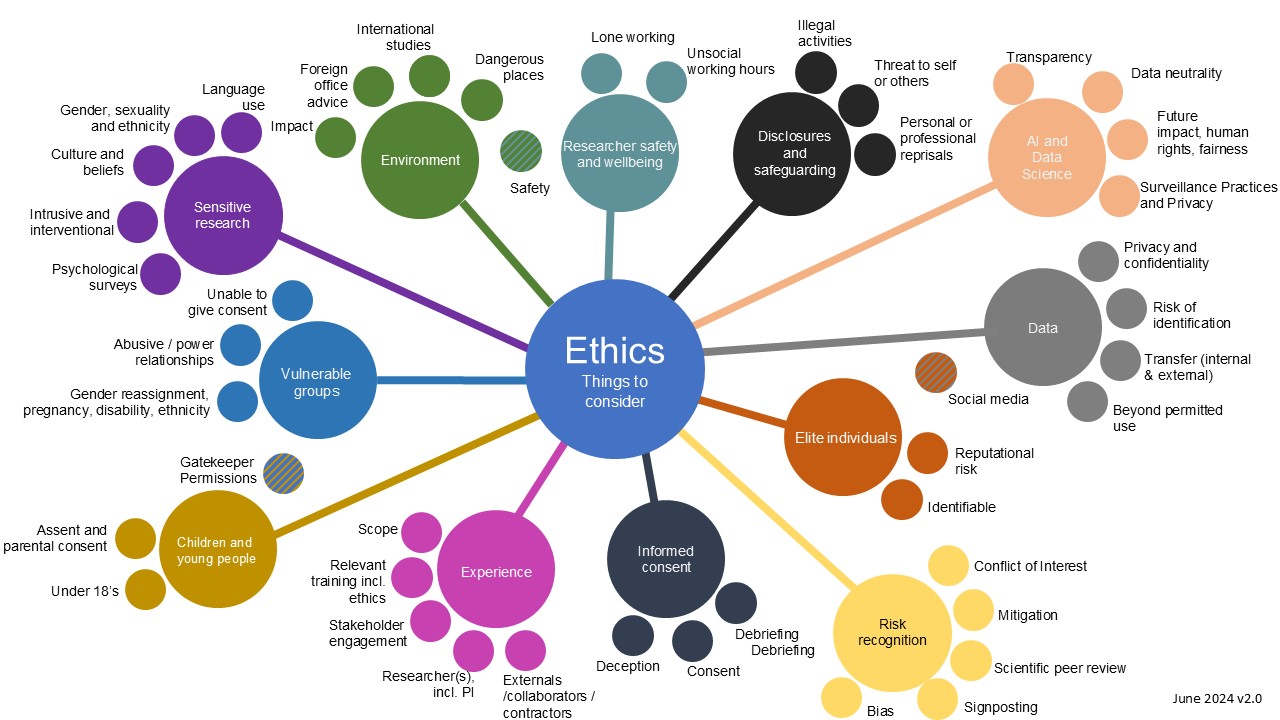
Figure 1: This infographic is to be used as an aid for identifying risks associated with your research.
This can include research on topics likely to evoke controversy in the community or strong emotional responses from participants.
Sensitive research requires Panel review, however if it is your area of expertise and you can show that you and/or members of your team have relevant experience within the area, this can act as a mitigator and may reduce the risk level.
Vulnerability may be defined several ways and may arise due to but not limited to the research participant being:
- unable to give informed consent
- in an abusive relationship
- in a power relationship
- in marginalised community
- in a protected characteristic group
- due to their personal experience of a traumatic event(s).
You need to assess potential vulnerability within the context of the research, in terms of potential consequences arising from their participation (immediate and long term) or lack of positive impact or personal benefits.
Research involving vulnerable groups will be of moderate risk level and require full Panel review. You must identify the risks and how you can mitigate them. For example, making changes to your research design, pre-screening to identify and eliminate high-risk participants, and providing participants with as much information as possible during informed consent, the development of safety monitoring plans and debriefing.
Research involving children and young adults under 18 years old will always be considered (at a minimum) moderate risk and require full panel review. Parent and/or Guardian consent is required in addition to participant consent. Age-appropriate participant information sheets should be used.
Any research involving children must balance the aims of the research, the safety and wellbeing of the children and their rights to participate in research. The same ethical questions that apply to adults also apply to children. By providing the right support and knowing when to take appropriate action, researchers can ensure that children feel respected and can participate safely.
These are interviews with senior people who may be chosen for inclusion in a research study because of the public role they hold (e.g., Government Ministers), or because they represent views of their general position (e.g., judges, newspaper editors). In most cases ‘elite’ interviews are considered moderate risk and would qualify for Panel review. This is because their privacy may be compromised as they may be unavoidably identifiable in the material generated (source: UKRI ESRC).
It is important to consider the nature of unequal relationships between the interviewer and interviewees and to put measures in place to address any associated ethical issues. Training is recommended for less experienced researchers. Researcher experience and appropriate training would mitigate some risks but would not necessarily downgrade the study to low-risk review.
Participants should be adequately and appropriately informed of potential risks arising from their participation in research to make a meaningful choice whether to take part in research or not.
Panel review is needed where participants take part in research without their full informed consent and where the full aims of the research are not disclosed prior to data collection. The definition of deception includes provision of false or misleading information, and the withholding of the whole truth about the aims of the study. Informed consent being a gold standard in research ethics, we would normally expect that a participant is fully informed of the true aims and objectives of the study in order that they can make an informed decision about whether they want to take part in the study. However, there are some studies that by their nature cannot do so as this would affect the integrity of the data and undermine the aims of the study (e.g., testing a new software program and the speed it takes participant to find the green button). In some cases, studies with a minor level of deception can be managed as low risk.
However, when a participant is not 'fully informed', this can lead to increased risk to the participant. The definition of deception includes provision of false or misleading information, and the withholding of the whole truth about the aims of the study (however with the latter the true aims). After the study has taken place, participants must be debriefed on the true nature of the study and reconsented.
Recognising risk is important for accurate risk analysis and better risk mitigation.
Researchers are responsible for ensuring that any risk to participants is minimised to the full extent possible before beginning research activities. Risk mitigation plans should identify the appropriate mitigation measures to reduce the likelihood of an identified (or unidentified) risk from occurring, and/or to lessen the impact in case it does happen.
Experience in or having research team members with experience within the research field under investigation is valuable. They provide insight into many aspects of conducting research ethically, the methodology, participants, data analysis, identification of risks and how to mitigate for these risks. Experience can act as a risk mitigator which potentially lowers the risk level.
For any research conducted outside your area of expertise, you must have an appropriate co-investigator / collaborator in the team. Students will need an appropriate supervisor(s). The lack of expertise can raise the risk level of the study or lead to the rejection of the ethics application.
The benefits of stakeholder engagement can include identifying, prioritising and refining topics for research; providing pragmatic feedback on the research protocol; aiding in recruitment of research participants; helping the researchers understand the participant’s perspective; ensuring that findings are interpreted with the end user in mind and that final products are readable and accessible; and facilitating wider dissemination and uptake of research findings.
Please refer to Queen Mary data information governance webpages for more information on records management and data protection. Data management alone cannot determine the risk level of the study, it must be assessed in conjunction with other aspects of your research. See also the Information/Data Governance Policy DG09 - Information Classification.
Library Services webpages contain useful information regarding research data management and curation at Queen Mary University of London.
For more information on how to transfer data between institutions please visit the JRMO contracts and agreements webpages.
Using social media data for research purposes raises numerous ethical concerns that researchers must address to uphold responsible and ethical practices. A common misconception is that social media data is freely available for researchers to use due to its public nature. However, in the case of most social media data, while they are technically available to access, it does not make them legitimately available for research purposes. Researchers are under the responsibility to respect the position of all the stakeholders involved in this data, including the platform and the user itself. This does not prevent researchers from using social media data altogether but requires them to take caution, especially when using information that could disclose the identity of the user i.e. direct quotes. It is important that proof of consent is first obtained in such cases. Additionally, researchers must consider the distinct terms of use applicable to each platform, as these can vary across different sites.
For further insight into this area, please find the webinar, ‘social media and ethics,’ by the UK Research Integrity Office, which discusses these issues in further depth.
Please also find a non-exhaustive list of the terms of use for various social media platforms:
Artificial intelligence (AI)technologies have the potential to re-shape the way we work, interact, and live. AI technology can benefit many areas, but without ethical safeguards, there is a risk of reproducing real world biases and discrimination.
Research considerations will include but not limited to:
- transparency of AI tools
- Human oversight
- Data privacy policies
- Copyright, Intellectual Property
AI and data science is an emerging and rapidly evolving field, expertise may need to be sought.
Disclosures of a sensitive nature or from a vulnerable group will require Panel review, however depending on the nature and how these disclosures are managed has the potential for review to be downgraded to a low risk.
You should assess the likelihood of the information disclosed becoming known and have a method for dealing with such situations prior to commencing the research.
Things to consider:
- The seriousness of the information.
- Are there any statutory requirements to disclose data collected?
- The impact not/disclosing the information will have and on whom.
- What support can be offered to participants?
- Will you need to inform someone other than the participant? If so, the participant may have to agree to this beforehand. What then happens if they refuse to give consent to you informing them?
Safeguarding, in the context of research, refers to the measures taken to ensure that all individuals that are employed on, participate in, or otherwise encounter research, do not come to harm because of their involvement, or because of the project's impact on their lives/communities. For more information, please see the safeguarding pages.
Researchers need to consider the potential risks to themselves and to others who may be involved with, or affected by, the research. Appropriate steps should be undertaken to mitigate these risks (e.g., undergoing a risk assessment process, implementing a lone work policy). The Health & Safety risk assessment policies and requirements are separate to research ethics; each School has a named Health & Safety Coordinator.
If you are travelling to a dangerous location for research purposes your study will be deemed as moderate risk as a minimum, this is regardless of whether you have been there before, or if it is your home.
Many countries require local ethical approval or registration of research projects, and some require specific research visas. QM Ethics team will always ask for evidence of the local REC approval (in your research site country) – if there is an appropriate one. You must abide by the local rules of the host country, or you may run the risk of legal action within the host country.
Working with local research teams can help mitigate many of the risks associated with conducting research abroad, potentially moving a moderate risk Panel review study to a low risk, Research Ethics team review.
If sanctions apply you will need to seek further information from the Queen Mary insurance office. Please note Queen Mary will not ensure studies proposed to take place within a country on the FCDO Red list.
Studies which potential cause adverse environmental impact would be deemed moderate risk (Panel review) or high-risk (Main Committee QMERC review). They have the potential to damage ecosystems, societies, and rare environments over the long term, but has the potential to bring both the University and the scientific process into disrepute, damaging our ability to initiate future research. As such, where research could (directly or indirectly) cause harm to the environment, you must ensure that the benefit of the research outweighs any risk and/or harm and must implement measures to reduce any risk/harm.
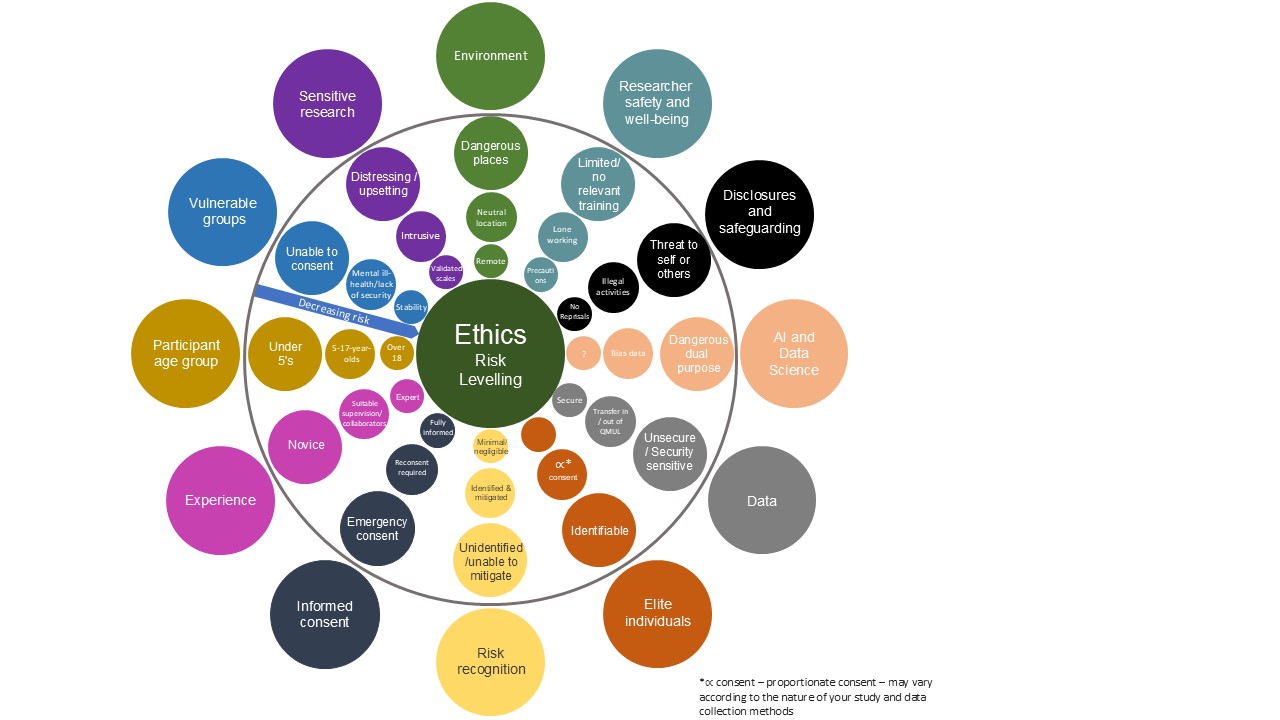
Figure 2: The types of risks and level of risk associated with each research study varies. The ‘Ethics: Risk levelling’ infographic serves as an example of how risk levels can increase or decrease.
